
Testing Summary
The main purpose of this page is to demonstrate the chemical compatibility of HP MJF PA12 with various fluids found in the automotive and general manufacturing industries as part of characterizing the technical feasibility of this material for fluid management applications.
Chemicals can affect visual, dimensional and mechanical properties. Therefore, properties such as tensile strength, Young’s modulus, elongation at breakpoint, weight variation and surface appearance have been evaluated in this study in order to evaluate for possible chemical attacks.
| Fluid | Mechanical | Mechanical | Mechanical | Visual check | Weight variation |
| Fluid | Tensile strength | Young modulus | Elongation at breakpoints | check | |
| Motor oil | Increased | Increased | Decreased | No Change | No Change |
| Valvoline Gear Oil | Increased | Increased | Decreased | No Change | No Change |
| Lubricating grease | Increased | Increased | Decreased | No Change | No Change |
| ATF Steering Fluid | Increased | Increased | Decreased | No Change | No Change |
| Brake fluid | No Change | Decreased | No Change | No Change | Increased |
| Antifreeze coolant (50%) | Decreased | Decreased | Increased | No Change | Increased |
In relation to mechanical properties:
- Motor oil did not show significant effects on PA12.
- Valvoline Gear Oil did not induce a reaction with PA12.
- ATF Steering Fluid did not attack or interact with PA12.
- Lubricating grease did not affect PA12.
- Brake fluid increased the weight of the sample by around 2%, the absorption of the brake fluid did not affect elongation, it did not react with PA12 as a plasticizer.
- Antifreeze coolant slightly plasticized the samples.
The following group of chemicals have been tested for general compatibility (non reactive) with HP PA12 but have not been tested for changes in the samples tensile strength, Young’s modulus, or Elongation at break.
| Fluid | Chemical resistance |
|---|---|
| Diluted alkalis | Good |
| Concentrated alkalis | Good |
| Hot water | Neutral |
| Chlorine salts | Good |
| Alcohol | Good |
| Esters | Good |
| Ketones | Good |
| Aliphatic hydrocarbons | Good |
| Aromatic hydrocarbons | Good |
| Toluene | Good |
| Unleaded gasoline | Good |
| Dot 3 brake fluid | Good |
| Chlorinated hydrocarbons | Neutral |
| Trichloroethylene | Neutral |
| shampoo (0.1% concentration in water) | Good |
| bleach (0.05% concentration in water) | Good |
| Methyl Ethyl Ketone (MEK) (24hr soak test)* | Neutral |
*Methyl Ethyl Ketone (MEK) is a powerful solvent used for cleaning in various industrial applications, especially those involving adhesives. After a 1 hour soak in MEK the PA12 parts show no changes, including part flexibility or strength. The same parts were then resubmerged in MEK for 24 hours and after removal and washdown showed minor plasticization on the fine detail as well as leaching of the black dye that had been applied to the parts prior to testing. No changes to the mechanical properties were observed.
Based on feedback from our customers who use Methyl Ethyl Ketone for long term continuous contact applications MEK will eventually start to break down the PA12 so it is not recommended for applications that will use it as a reservoir for MEK. But MEK can be used to clean PA12 parts without any issues.
In conclusion, the chemical compatibility of HP MJF PA12 with these fluids seems to be acceptable as no sample showed evidence of chemical reactions, stress cracking, or solvation.
Chemical Compatibility of HP MJF PA12 Testing details
Visual check performance summary:





- No discoloration or color darkening occurred due to contact with any fluids. After complete drying, all the tensiles recovered their original colors. Therefore, no color impact occurred, at least not with the 4-week duration at 140°F.
- No surface corrosion was seen due to any of the fluids, as all samples show their original colors. After drying, the initial colors were recovered in all the tests.
- No visual stress cracking was seen on the surface of the tensiles.
Mechanical Testing:
In a chemical interaction, one of the main consequences is interference with the polymer chains of the given plastic, which likely reduces its mechanical properties. To determine any possible changes in the mechanical behavior of the tensiles that were in contact with the fluids in the climatic chamber or possible solvation or plasticization, a tensile test was performed, evaluating the tensile strength, the Young’s modulus and the elongation at breakpoints of the samples. The following charts show the results each mechanical property for the fluids tested in comparison with the control zone created between the control group (the orange line) and the datasheet values (the blue line).
Tensile strength:
The tensile strength of a material is the maximum amount of tensile stress that it can withstand before failure (for example: breaking).
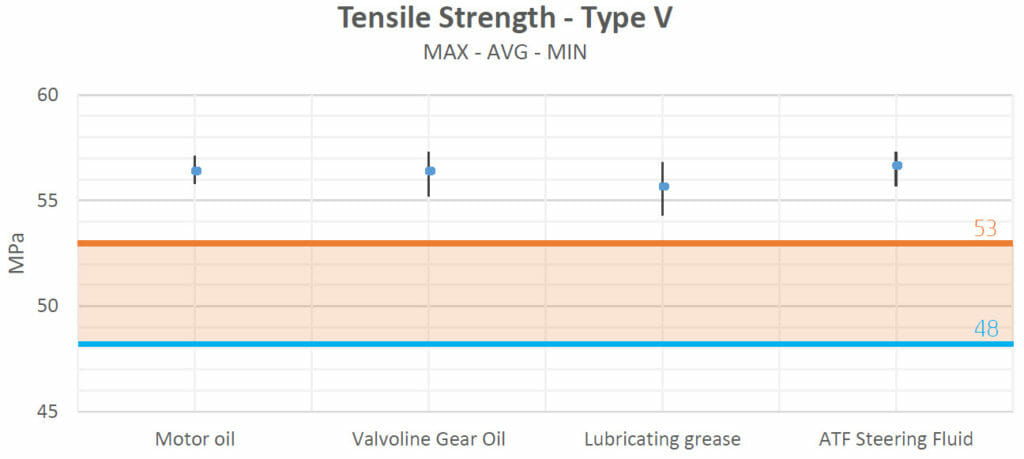
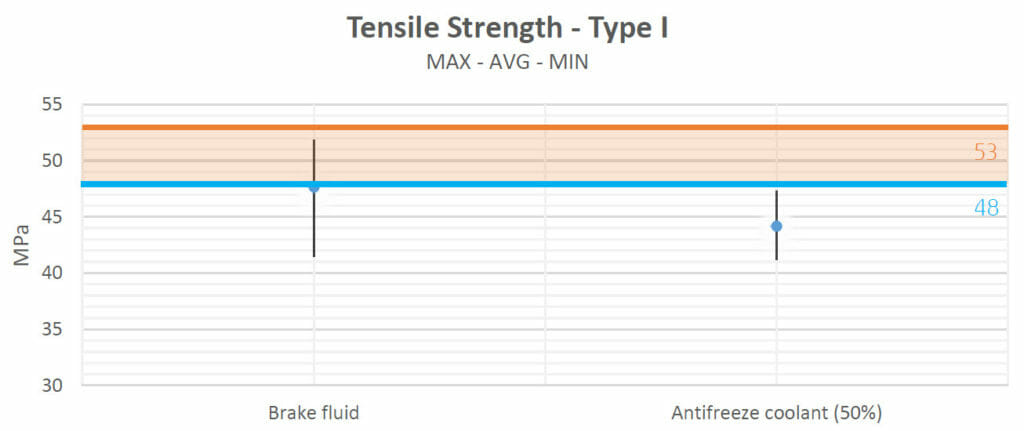
The tensile strength values obtained after the heating process in test 1 show that there were no negative interactions with most fluids (motor oil, Valvoline Gear Oil, lubricating grease and ATF Steering Fluid). No variations seemed to appear when the samples were in contact with brake fluid either. Only with antifreeze did the strength of the samples decrease.
Young’s modulus:
Young’s modulus characterizes how difficult it is to deform a material in terms of elastic behavior. As in the previous subsection, the following charts show the test values obtained with the datasheet (the blue line) and the control group values (the orange line).
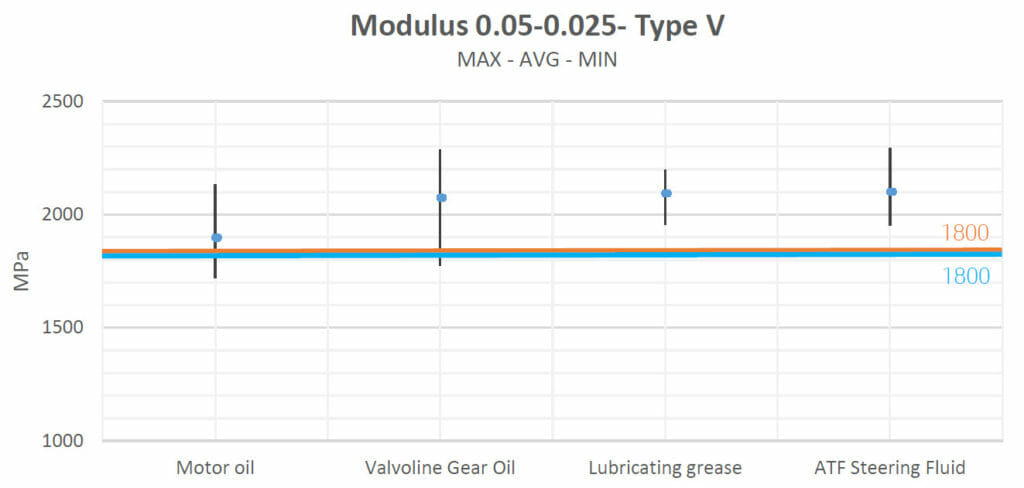
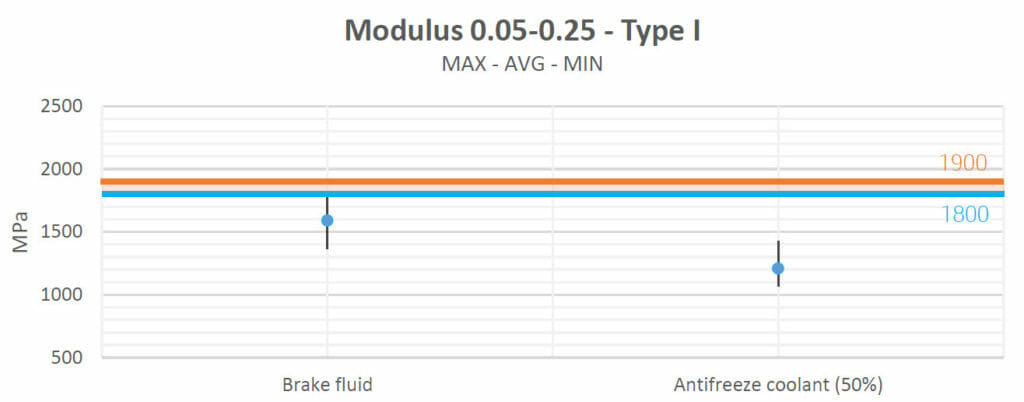
Motor oil, Valvoline Gear Oil, lubricating grease and ATF Steering Fluid increased the materials’ strengths and their Young’s moduli slightly with respect to their natural values.
Brake fluid and antifreeze decreased the materials’ Young’s moduli.
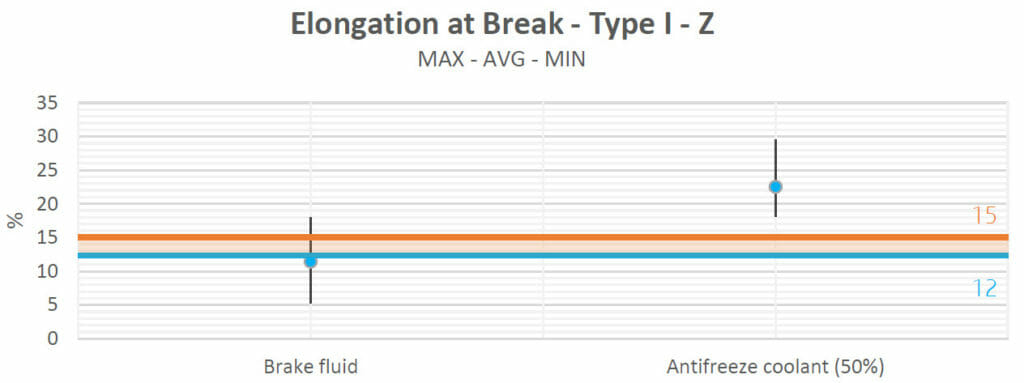
Elongation at breakpoints:
The elongations at breakpoints were studied in order to evaluate whether the fluids had any effect on the plastic behavior of the material. Plasticization tends to soften polymers, increasing their ductility and thus causing an increase in elongation, while at the same time lowering their tensile strengths and Young’s moduli.
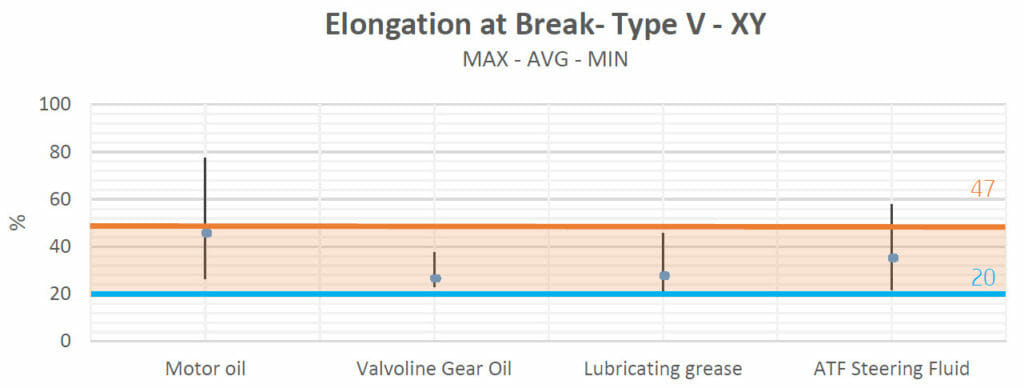
Concerning Valvoline Gear Oil, lubricating grease and ATF Steering Fluid: Elongation values were between the control group values and those of the datasheets.
As can be seen, the average value for the tensiles which were in contact with motor oil was also in the control zone. The main difference with the other results was the range of the values obtained, which was wider compared to the results of the other groups and reached a maximum near 80% of elongation. This high variability could be due to the measurements of the type-V tensiles. However, as the average value is under the baseline and in the control zone, we can consider that contact with motor oil does not have a significant effect on this mechanical property.
Brake fluid did not cause any alterations to the samples (in terms of elongation) either.
Antifreeze produced an increase in the elongation, which reaffirms the natural behavior because the tensile strengths and Young’s moduli both decreased. They were inverse to the elongation at breakpoint. If all the properties increased or decreased at the same time, this would mean that a sure chemical reaction had occurred, but this is not the case, as only a plasticizing phenomenon took place. This will be confirmed in the weight absorption analysis.